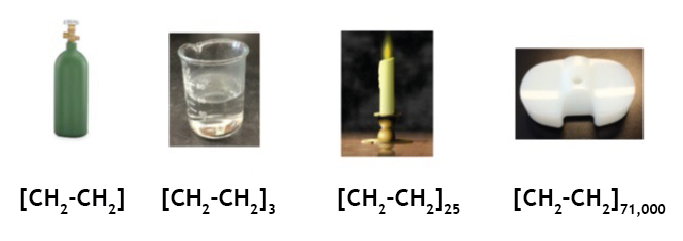
Ethylene gas, hexane, paraffin wax, and polyethylene all have the same chemical building block, namely an ethylene group (CH2-CH2). The difference in properties in these materials comes from the number of ethylene groups that make up a single molecule. Ethylene gas has a single group (or repeat unit), hexane has 3, and polyethylene can go up in excess of 70,000 repeat units. As the number of repeat units increase, the molecule moves from the idea of a rigid linear rod to a floppy random coil. When placed with other ethylene molecules of comparable size, the chains entangle, which causes the material to transition from a liquid to a solid, as the entangled chains resist disentanglement until sufficient energy, usually in the form of heat, is applied. Some portions of the chains will also align to form crystal structures, which further solidify and rigidify the material.
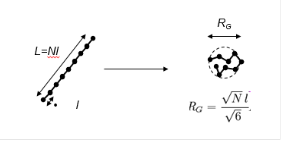
Although some techniques exist to count the number of repeat units in a material, such as MALDI, molecular weight is normally determined by inferred techniques that look at the size of the individual polymer chain in solution. The size of the chain depends both on its molecular weight and the solvent environment it is placed in (a better solvent and improved temperature will tend to expand the polymer chain more). As shown below, a rigid rod molecule’s size is the number of repeat units multiplied by the length of each repeat units. Most polymers will occupy space as a coil, shown on the right, with a size (the radius of gyration, Rg) dictated by the equation shown below for a good solvent.
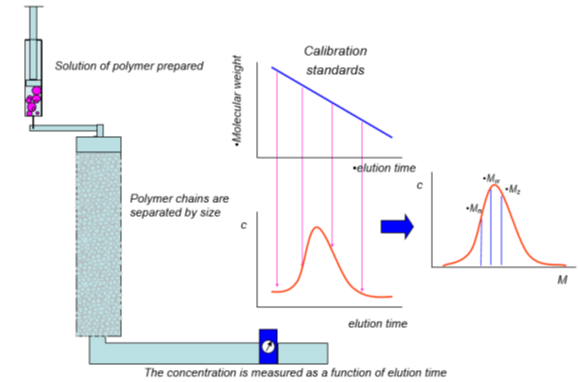
Researchers have derived relationships between measured properties of polymer solutions, such as viscosity, light diffraction, and osmotic pressure, to determine molecular weights. These techniques all report a mean value of molecular weight. Most polymers have a distribution of molecular weights, as the polymerization process will yield shorter and longer chains around the nominal desired chain length. Gel permeation chromatography (GPC) is a technique that allows determination of the distribution of molecular weights.
In GPC, a dilute solution of polymeric material is prepared using a good solvent for the polymer. This solution is passed through a chromatography column that contains a porous structure that is sized to allow penetration of smaller polymer chains while excluding the larger ones, which effectively separates the polymer chains by size as they exit the column. A detector (or multiple detectors) measures the quantity of the polymer chains at each elution time, forming a concentration vs. elution time plot. A series of calibration standards are injected through the same column, and the molecular weight vs. elution time is determined. By converting elution time to molecular weight for the unknown sample, a molecular weight distribution plot can be determined. As may have been obvious, the actual molecular weights are not measured; rather, the size of the polymer chains are, with the assumption that the size scales with molecular weight.
CPG provides GPC analysis on a variety of polymer types, including water soluble, organic soluble, high temperature (for polyolefins), and biologic materials. We can also perform testing using viscometry detectors and light scattering.